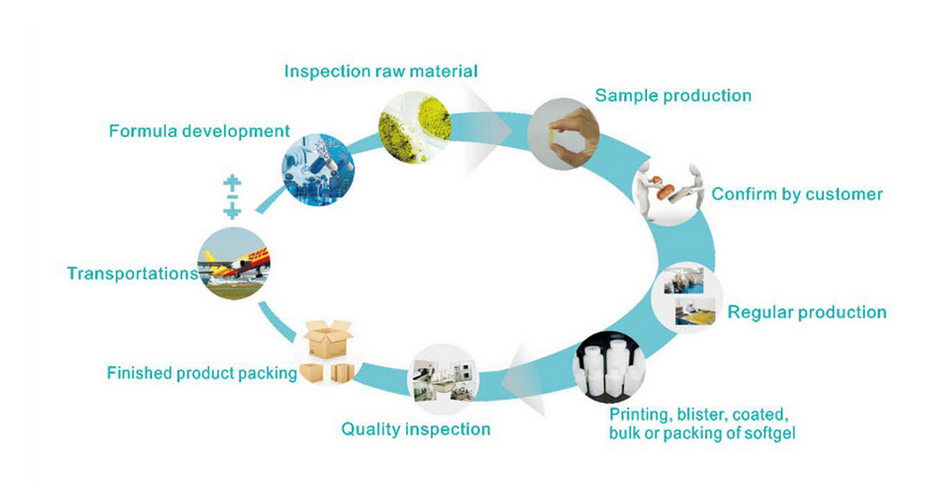
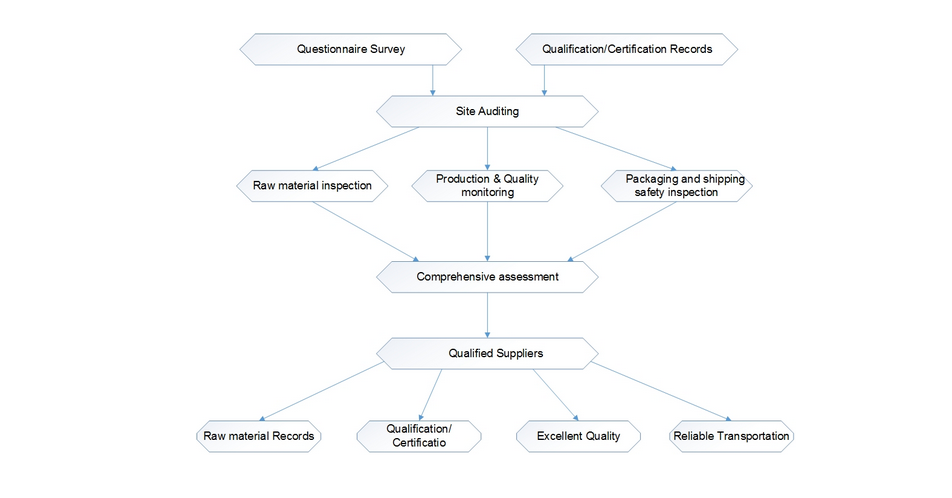
A quality management team with appropriate and sufficient education background as well as necessary qualifications in pharmaceutical fields is established and well-involved in all the production activities, in terms of Quality Assurance (QA) and Quality Control (QC).
The Quality Control analysis lab is installed with necessary and adequate as well as validated facilities and means for quality examination of materials. The entire flow of goods from raw materials, intermediates, process water, etc., to end products are monitored to ensure the conformity of whole production activity.
The quality management system is established based upon “ICH Harmonized Tripartite Guideline — Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients Q7″ (ICH-Q7A). Nevertheless, the system is keeping improved by a series of deficiency-finding and corrective actions, including regular internal audit, clients’ audit and authority inspections.